Flipping the script on fungal infections
January 12, 2026
Fungal infections are a serious public health concern that cause millions of deaths each year.
Among the most worrying are Candida auris and Cryptococcus neoformans, two fungi that can lead to severe conditions like meningitis, pneumonia and nervous system disorders, especially in people with weakened immune systems.
Although most fungi struggle to survive at temperatures above 86 degrees Fahrenheit — roughly the warmth of human skin — these two pathogenic fungi are exceptions. Candida auris and Cryptococcus neoformans thrive at 98.6 degrees Fahrenheit, the internal temperature of the human body, which allows them to cause serious infections.
“Fungi spread via spores that are released into the environment. For immunocompromised people, these infections can be extremely challenging to treat,” said Huilin Li, Ph.D., the Ralph and Grace Hauenstein Chair in Structural Biology at Van Andel Institute. “Once they find their way into the bloodstream, they can cause severe damage throughout the body, including the eyes, kidneys, heart, lungs and brain.”
Despite the prevalence of fungal infections, treatment options remain very limited and often are toxic to the human body. To make things more complicated, many fungi, including Candida auris and Cryptococcus neoformans, are growing increasingly resistant to existing therapies.
Acquired resistance can develop for several reasons including improper medication use, spontaneous resistance (caused by random genetic mutations) and transmitted resistance, where resistant strains spread from one person to another.
Understanding why resistance develops is only one part of the solution. Scientists like Li, who study how the structure of molecules inform their function, also are searching for new treatment options that target drug resistance in fungi’s defenses.
Recent findings published in Cell, may steer us in the right direction.
A team effort to improve antifungal treatments
In their new study, a team led by Li and McMaster University’s Gerard Wright, Ph.D., identified a natural substance called butyrolactol A, which may help a group of low-toxicity antifungal drugs known as echinocandins work against drug-resistant fungal infections including Candida auris and Cryptococcus neoformans.
Echinocandins are one of three main types of medications used to treat fungal infections. They generally have mild side effects, and work by inhibiting a key enzyme involved in building the fungal cell wall, effectively halting fungal growth and leading to cell death.
However, like other anti-fungal drugs, the rise of resistance has limited their effectiveness as a stand-alone treatment.
“Finding a way to improve low-toxicity medications — which are easier on patients because they have fewer side effects — is critically important,” Li said. “Part of the problem with current treatments is that, while they are effective, they also can have severe side effects that limit how long and how aggressively we can treat the infection. Moreover, Cryptococcus neoformans causes a type of meningitis that is inherently resistant to echinocandins. If we can improve their effectiveness, we may be able to overcome that resistance.”
Breaking it down to break through
Li and Wright’s teams did more than just identify butyrolactol A — they figured out exactly what it does.
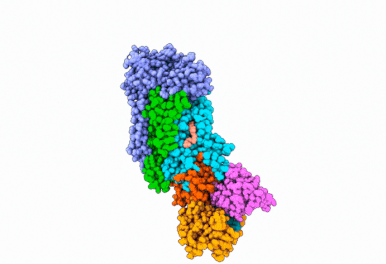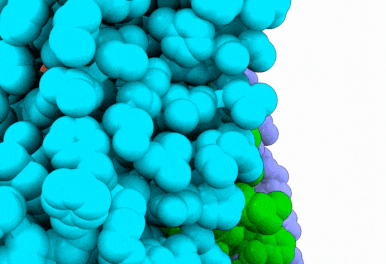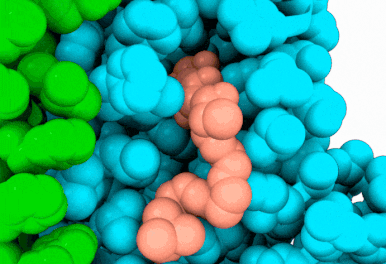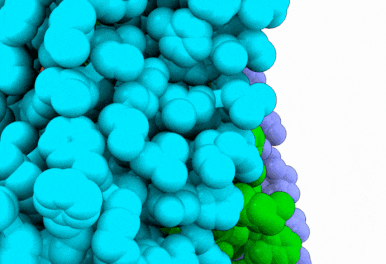
Butyrolactol A works by blocking a lipid transport protein called flippase Apt1–Cdc50 in Cryptococcus neoformans. Normally, this flippase helps distribute lipids in the cell membrane by moving them from outside of the cell to the inside. This carefully orchestrated process supports key aspects of cellular function, including membrane construction, protein transport and cell communication. By inhibiting the flippase, butyrolactol A interferes with the fungus’s internal environment, allowing the antifungal drug echinocandin to get inside more easily — thus making the treatment more effective.
“Thanks to our state-of-the-art Cryo-EM Core, we were able to reveal how the new antifungal compound butyrolactol A binds to its target,” said Diessel Duan, Ph.D., a research scientist in the Li Lab and co-first author of the study. “These findings lay the groundwork for designing more effective antifungal drugs.”
The team’s prior work on flippases in model fungus S. cerevisiae, or baker’s yeast, set the stage for this discovery by improving their understanding of how fungal resistance operates at the molecular level.
“Fungal infections are evolving, so our strategies must advance faster,” said Li. “By revisiting natural compounds to enhance the efficacy of echinocandins, we’re one step closer to developing stronger, more resilient antifungal therapies.”
Funding Acknowledgement
Research reported in this publication was supported by the Canadian Institutes for Health Research under award nos. PJT190298 (Wright), FRN143215 (Brown), MOP119391 (Truant), PJT156067 (MacNeil) and FDN154288 (Cowen); the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award nos. R01AI039115 (Heitman), R01AI050113 (Heitman), R01AI170543 (Heitman), R01AI172451 (Heitman), R01AI133654 (Heitman and Magwene), R01AI127375 (Cowen), F31AI150120 (Hoy) and R01AI053721 (Kronstad); the National Cancer Institute of the National Institutes of Health under award no. R01CA231466 (Li); and Van Andel Institute (Li).
Cowen is a Canada Research Chair (Tier 1) in Microbial Genomics & Infectious Disease and co-director of the Canadian Institute for Advanced Research (CIFAR) Fungal Kingdom: Threats & Opportunities program (CIFAR Catalyst Grants on BLA: CP21-065; Cowen [University of Toronto]; Heitman [Duke University]; Wright [McMaster University]; and Boone [University of Toronto]).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders.

