Research brief: Searching for a crystal-clear picture of a molecular complex linked to cancer, other diseases
June 5, 2017
Protein: As the workhorses of biology, proteins are involved in virtually every process in the body, from copying the genetic code to digestion.
Receptor: Receptors function much like antennae. They are special proteins that receive chemical signals from the environment and convey the information to the cell through a cascade of reactions.
Wnt: A group of proteins that play critical roles in a wide range of biological processes from embryonic development to cancer.
Wnt signaling: A cellular communication pathway moderated by Wnt proteins. The Wnt signaling pathway is a prominent target for treating diseases such as cancer.
Frizzled: A group of receptors that interact with Wnt proteins, particularly in development.
Dimerization: A reaction that causes two individual units to tightly associate with other.
Oligomerization: A process that causes monomers (molecules made up of one unit, such as individual proteins) to form longer chains of molecules, such as dimers
New images have revealed previously undiscovered facets of a key player in a complex cellular communication network, potentially paving the way for more effective treatments for a host of diseases.
The findings center on the Wnt signaling pathway, an intricate web of molecules and chemical reactions responsible for conveying biological messages related to development and stem cell maintenance, and whose dysregulation is associated with many cancers. Given its immense size and role in dozens of processes in the body, the Wnt pathway has long been a target of drug development efforts for the treatment of cancer, osteoporosis and many other conditions.
Biologists often say structure informs function, meaning that the shape and make up of a molecule is closely related to its role. In the case of signaling, receptors nest in the cell membrane, with one end protruding into the cell’s environment. Signaling proteins, such as Wnt, dock at these receptors, setting off a cascade of reactions that convey messages to the cell. The availability or non-availability of these docking areas determine if and when signals are transmitted.
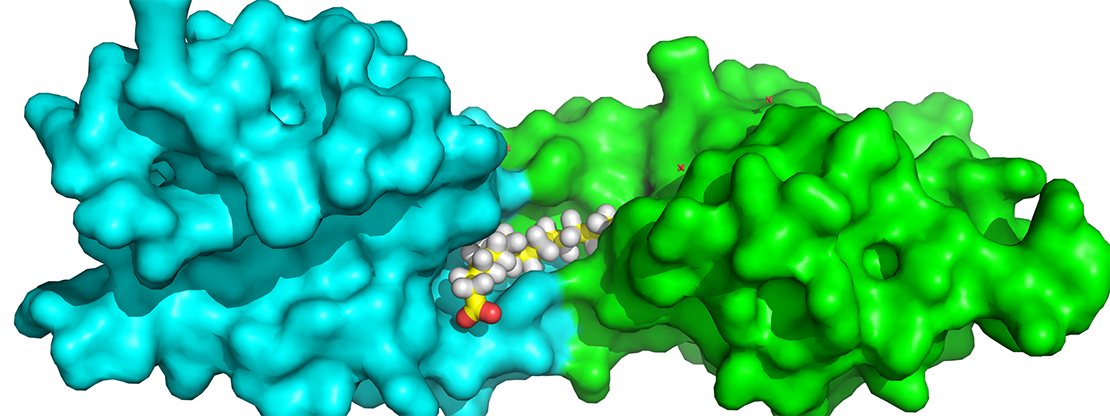
However, the details of how the interaction between Wnt proteins and their receptors, a group of molecules called Frizzled receptors, triggers signaling has largely remained a mystery. Now, thanks to researchers from Van Andel Research Institute and colleagues from Mayo Clinic and University of Washington, a clearer picture has emerged.
“Our findings further explain how Wnt proteins activate their receptors and provides a more complete model for how this process works both outside and inside the cell,” said Karsten Melcher, Ph.D., an associate professor at VARI and a senior author on the study. “There is still much to learn about how Frizzled receptors transmit Wnt binding signals across the cell membrane. Our structure will provide needed insight for further study and, hopefully, the development of more efficient treatments that target the Wnt pathway.”
Wnts are secreted proteins that are modified by a fatty acid, which in turn directly links up with Frizzled receptors. Using a technique called X-ray crystallography, the team imaged the structure of one of these receptors—Frizzled-4—while it was bound to the fatty acid. Unexpectedly, they found that the fatty acid simultaneously binds to the outside of two receptors, linking them on either side of the cell membrane to initiate signaling, much like a wire in a circuit. Similar results were independently reported by another research group. Much as a key is designed to fit a lock, a small-molecule drug could be developed that interacts with the receptor in place of Wnt, potentially acting as a potent therapy for a number of diseases.
“Take colorectal cancer as an example—almost all cases are associated with abnormal Wnt signaling,” said H. Eric Xu, Ph.D., a professor at VARI and a senior author, said. “More insight equals more impact. Understanding what’s occurring at the most basic level is the first step in finding new therapies.”
The implications are far-reaching and even go beyond the Wnt pathway. Frizzled receptors belong to a family of molecules called G protein–coupled receptors, which are targeted by about 30 percent of drugs currently on the market. Scientists are racing to tap into this potential therapeutic goldmine, along with other pathways and molecular families related to it in hopes of finding life-changing treatments.
Although the crystal structure of this complex has provided needed insight, Melcher, Xu and his colleagues want to take it a step further and they’re looking to VARI’s new suite of powerful cryo-electron microscopes to do it. Called cryo-EM for short, this technology allows scientists to see tiny molecules in their natural state at atomic resolution—down to 1/10,000th the width of a human hair. This endeavor is often difficult or even impossible with X-ray crystallography, which requires scientists to painstakingly coax proteins into crystalline structures before they can be imaged.
This work was a collaboration between the Melcher, Xu and Williams laboratories at Van Andel Research Institute, and colleagues at Mayo Clinic and University of Washington.
Want to read more? The paper is available online in Genes and Development here.
DeBruine ZJ, Ke J, Harikumar KG, Gu X, Borowsky P, Williams BO, Xu W, Miller LJ, Xu HE, Melcher K. 2017. Wnt5a promotes Frizzled-4 singalosome assembly by stabilizing cysteine-rich domain dimerization. Genes Dev.