Diabetes drug slows experimental Parkinson’s disease progression, human trials to begin next year
December 7, 2016
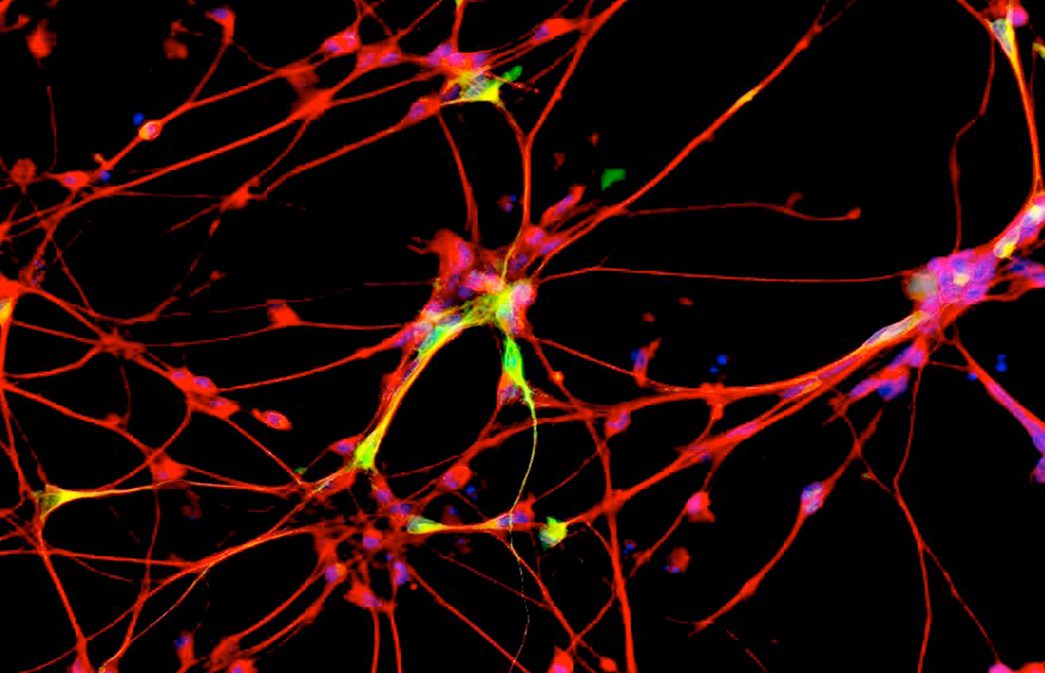
New data published today shows drug preserves critical brain function in laboratory
models of Parkinson’s
GRAND RAPIDS, Mich. (Dec. 7, 2016)—A new investigational drug originally developed for type 2 diabetes is being readied for human clinical trials in search of the world’s first treatment to impede the progression of Parkinson’s disease, following publication of research findings today in the journal Science Translational Medicine.
“We hope this will be a watershed moment for millions of people living with Parkinson’s disease,” says Patrik Brundin, M.D., Ph.D., director of Van Andel Research Institute’s Center for Neurodegenerative Science, chairman of The Cure Parkinson’s Trust’s Linked Clinical Trials Committee, and the study’s senior author. “All of our research in Parkinson’s models suggests this drug could potentially slow the disease’s progression in people as well.”
Until now, Parkinson’s treatments have focused on symptom management. If successful in human trials, MSDC-0160 would be the world’s first therapy to treat the underlying disease and slow its progression—potentially improving quality of life and preventing the occurrence of falls and cognitive decline. It may also reduce or delay the need for medications that can have debilitating side effects, says Brundin.
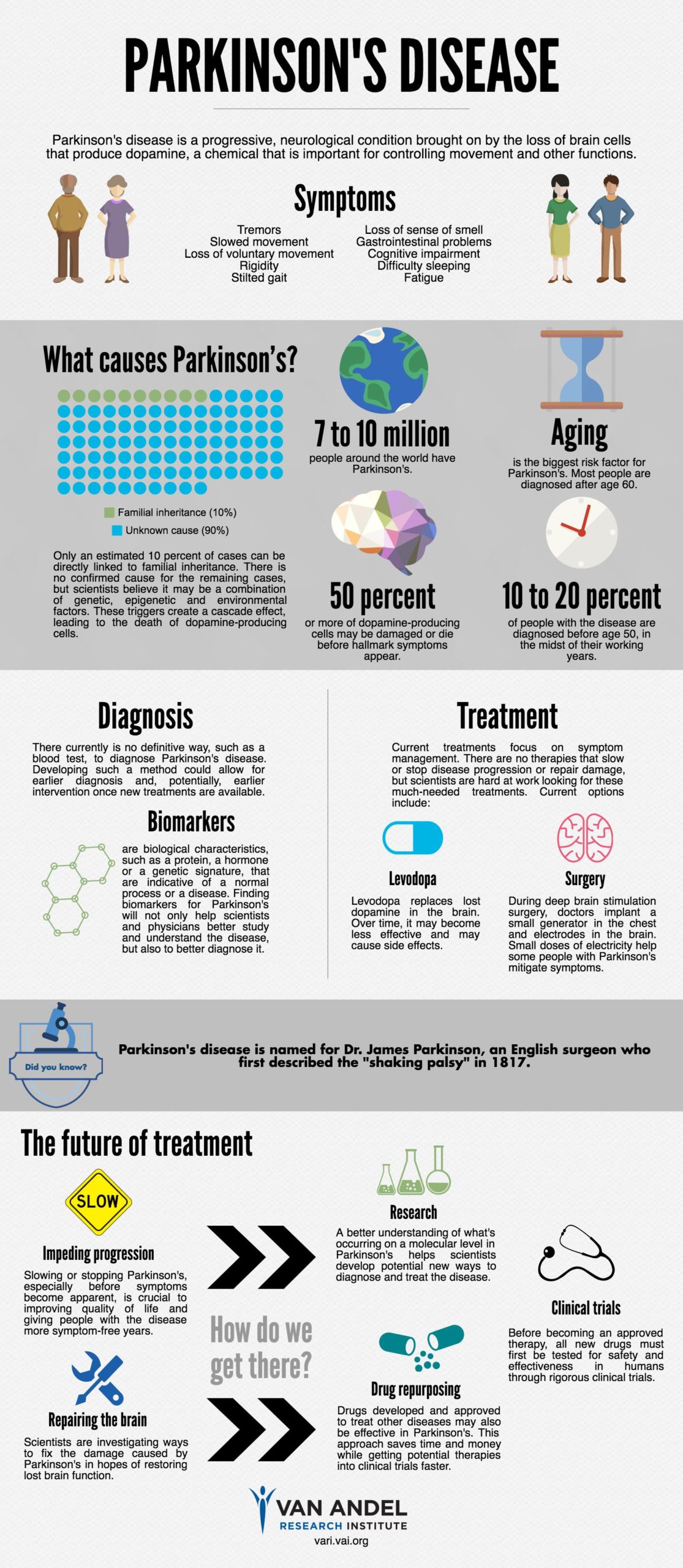
Parkinson’s disease afflicts between 7-10 million people worldwide, including an estimated 1 million Americans, and these numbers are expected to increase dramatically as the average human lifespan increases. There is currently no cure, and first-line treatment has remained relatively unchanged since the introduction of levodopa in the 1960s.
Tom Isaacs, a co-founder of The Cure Parkinson’s Trust who has lived with Parkinson’s for 22 years, says MSDC-0160 represents one of the most promising treatments the Trust’s international consortium has seen to date.
“Our scientific team has evaluated more than 120 potential treatments for Parkinson’s disease, and MSDC-0160 offers the genuine prospect of being a breakthrough that could make a significant and permanent impact on people’s lives in the near future,” says Isaacs. “We are working tirelessly to move this drug into human trials as quickly as possible in our pursuit of a cure.”
MSDC-0160 was developed by Kalamazoo, Michigan-based Metabolic Solutions Development Company (MSDC) to treat type 2 diabetes. In 2012, Brundin recognized it as an exciting drug candidate because of its mode of action, proven safety in people, local availability and the start-up company’s interest in collaborating on drug repurposing initiatives. After four years of work, the effects of the drug in the laboratory exceeded Brundin’s expectations.
The novelty of MSDC-0160 stems from a recently revived revelation that Parkinson’s may originate, at least partially, in the body’s energy metabolism. The new drug appears to regulate mitochondrial function in brain cells and restore the cells’ ability to convert basic nutrients into energy. Consequently, the cells’ ability to handle potentially harmful proteins is normalized, which leads to reduced inflammation and less nerve cell death.
“Parkinson’s disease and diabetes may have vastly different symptoms with unrelated patient outcomes; however, we’re discovering they share many underlying mechanisms at the molecular level and respond similarly to a new class of insulin sensitizers like MSDC-0160,” says Jerry Colca, Ph.D., co-founder, president and chief scientific officer of MSDC.
While Brundin says he is eager to see MSDC-0160 launched into a clinical trial in Parkinson’s disease, he’s equally excited about the possibility of testing the drug in Lewy body dementia and other cognitive decline conditions, such as Alzheimer’s disease.
“This is an immensely promising avenue for drug discovery,” says Brundin. “Whatever the outcome of the upcoming trial for Parkinson’s, we now have a new road to follow in search of better treatments that cut to the root of this and other insidious diseases.”
The Cure Parkinson’s Trust and Van Andel Research Institute are currently working with MSDC to address regulatory issues and obtain funding to organize the clinical trial, which Brundin hopes can begin sometime in 2017.
Funding for the research was provided by Van Andel Research Institute, The Cure Parkinson’s Trust, the Campbell Foundation and the Spica Foundation.
The paper’s authors include Anamitra Ghosh, Trevor Tyson, Sonia George, Erin N. Hildebrandt, Jennifer A. Steiner, Zachary Madaj, Emily Schulz, Emily Machiela, Martha L. Escobar Galvis, Jeremy M. Van Raamsdonk and Patrik Brundin, all of Van Andel Research Institute; William G. McDonald and Jerry R. Colca, both of Metabolic Solutions Development Company; and Jeffrey H. Kordower, of Rush University Medical Center and Van Andel Research Institute.
# # #
About Parkinson’s disease
Although Parkinson’s symptoms vary widely among patients, they typically progress from a constellation of vague problems, including loss of smell, constipation, poor sleep, mood fluctuations, and changing blood pressures, to the disease’s hallmarks, such as tremors, affected speech and facial expressions, stooping, poor motor function, and memory loss.
The gold standard treatment, levodopa, supplements the neurotransmitter dopamine, a chemical in the brain that is critical for motor function and one that decreases as the disease progresses. Though often effective early in Parkinson’s progression, levodopa’s effectiveness may decline and the need to endure increasingly heavy doses comes with debilitating side effects like confusion, anxiety, hallucinations, uncontrollable movements and tremors, and incontinence.
About MSDC-0160
MSDC-0160 is a novel mTOT modulator being developed to treat neurodegenerative diseases such as Parkinson’s and Alzheimer’s disease. It is a once-a-day, oral insulin sensitizer that works through a new drug target located in the inner mitochondrial membrane, called mTOT. The mTOT mechanism ties mitochondrial metabolism to aspects of the pathology associated with insulin resistance and certain neurodegenerative diseases.
MSDC-0160 has been shown to safe, well-tolerated, and efficacious in a 90-day, randomized, double-blind, comparator- and placebo-controlled, multi-dose Phase 2b clinical study in 258 patients with type 2 diabetes (Clinical Pharmacology & Therapeutics (advance online publication 6 March 2013); and in a Phase 2a study conducted by researchers at Rush University Medical Center in Chicago in patients diagnosed with dementia due to Alzheimer’s disease, wherein this new therapeutic agent prevented the decline in a measure of brain glucose utilization in patients with mild to moderate Alzheimer’s disease (Current Alzheimer Research, 2014, 11).
Read Dr. Brundin’s blog post “A New Hope for Parkinson’s” by clicking here.
About Parkinson’s Disease
MEDIA CONTACT:
Beth Hinshaw Hall, 616.234.5519, [email protected]