How the smallest components of life work together to manage our epigenetic inheritance
When it comes to understanding the intricate genetic architecture that makes us human, the old adage holds true — the whole is greater than the sum of its parts.
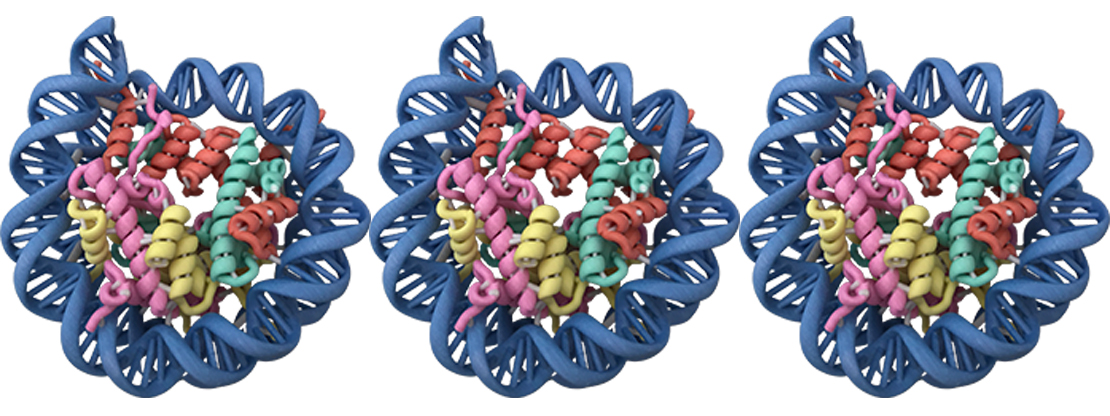
That’s according to new findings published recently by Van Andel Research Institute’s Dr. Scott Rothbart and his team showing the minute molecular components that regulate our epigenetic inheritance are much more intertwined and dependent upon each other than previously thought.
Robert Vaughan, a Van Andel Institute Graduate School Ph.D. student in the Rothbart Laboratory and first author on the study, likened their approach and results to a mechanic working on a vehicle.
“If you want to know about a car but you separately study the tires and the steering wheel, you’ll miss how individual components function together to create a driveable unit,” he said. “The same is true with biology.”
The study highlights a surprising deviation from a major tenet of biology called “the central dogma,” which outlines how our genetic information flows in one direction — from the DNA to a messenger molecule called RNA and finally to proteins, molecular workhorses that are responsible for making each and every process in the human body happen.
They found that under the right circumstances, this stream of information can bypass RNA altogether, a discovery with implications not only for better understanding our biology, but also for developing new medications for cancer and many other diseases.
“One of the most exciting things is our findings show that DNA directly controls the behavior of a family of proteins, something we may not have seen had we not been looking at multiple behaviors of the proteins simulteneously,” Vaughan said. “While this interaction certainly doesn’t happen all of the time, we’ve identified a situation where the flow of genetic information skips a step, which could help us gain a clearer understanding of what’s happening in the background of health and disease.”
The reason for this apparent leap-frog? A second layer of control called epigenetics that regulates how the instructions in the DNA are read and expressed through chemical tags placed directly on the genome. Although epigenetic mechanisms and their roles in regulating genetic information have been studied for years, this is the first time a particular epigenetic tag has been shown to directly alter protein function.
Curious about epigenetics? Check out our video explainer.
At the center of their work is a protein called UHRF1 that ensures layers of epigenetic information on our DNA is correctly passed from one generation of cells to the next. In 2016, Rothbart’s team revealed how UHRF1 works with other epigenetic processes to carry out its job of “reading” molecular signals from the DNA and “writing,” or adding special epigenetic tags, to DNA, ultimately ensuring the integrity of our genetic material. The level of crosstalk and cooperation between the different pieces and systems revealed by both studies was remarkable, Rothbart said.
“Our findings add an entirely new layer to an already complicated regulatory system,” he explained. “DNA, and the way DNA is marked with epigenetic tags, is responsible for who we are, from our height to our predisposition to diseases like cancer. The revelation here is that different types of epigenetic tags — these tiny chemical marks on the DNA — can change the function of a protein in a much more direct way than previously thought, which has an impact on our physiology.”
Just like a mechanic learning his craft, the challenge the team now faces is sorting out how the car actually gets put together; that is, determining how all of these tiny pieces fit to make a functional, cohesive whole.
Previously, Vaughan and Rothbart said, they’ve taken a reductionist approach, distilling genetic and epigenetic components into their most basic forms, which helps remove variables that may obscure critical molecular interactions. Now, they plan to move from the test tube back into the cell, which will allow them to investigate the flow of genetic information in more complex systems. While this admittedly complicates things, the potential payoff, they say, is worth it.
“Epigenetic tags are often out of sorts in cancer and other disorders,” Vaughan said. “Understanding the proteins that directly interact with these tags may reveal some vulnerability that can be targeted for treatment.”
In addition to Vaughan and Rothbart, authors include Dr. Brad Dickson and Dr. Evan Cornett of VARI, and Dr. Joseph Harrison and Dr. Brian Kuhlman of University of North Carolina at Chapel Hill. The study was published in Nucleic Acids Research (doi:10.1093/nar/gky151).
Research reported in this publication was supported by the National Institute of General Medical Sciences of National Institutes of Health under Award Number GM124736 and the Van Andel Institute Graduate School. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.