A genetic program, not an accumulation of damage, may be responsible for aging
April 14, 2016
They’re fundamental human questions—how do we age? And why?
A long-accepted theory holds that damage accumulated through time—from sun exposure and other environmental factors, for example—leads to aging but recent scientific findings are challenging this paradigm. Among them is a newly published paper from Van Andel Research Institute’s Dr. Jeremy Van Raamsdonk that provides evidence for a different theory, one that is linked to genetics rather than a stockpile of damage.
What is stress?
Before we get into the details, let’s examine a basic question—what is stress? We’ve all experienced it—stress at work, stress from classes or stress from strained conversation with a friend. In biological terms, stress is defined as the psychological and biological response to a real or perceived demand or threat. When a stressful event occurs—say you found out a project deadline got moved up by a month—a biological cascade goes into effect. First, the body judges whether the event is stressful or not based on sensory cues and memories (remember what happened last time a deadline got moved up?). A part of the brain known as the hypothalamus then kicks into gear leading to the production of stress hormones, which have a host of effects including increased heart rate and breathing. These responses prepare you for fight or flight—that is, to deal with the cause of your stress or to flee from it. Although stress (and the body’s response to it) serves an important purpose, prolonged stress can have serious health implications such as high blood pressure and heart disease.
What did they find and how did they do it?
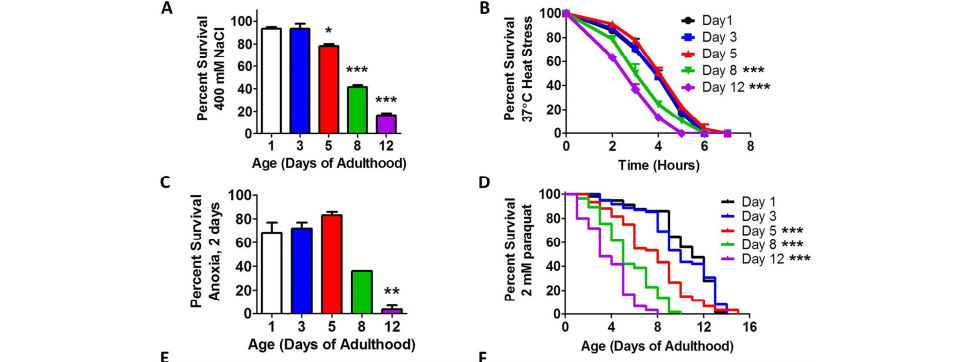
Back to Van Raamsdonk’s findings. Using a tiny worm called Caenorhabditis elegans as a model, Van Raamsdonk and his team found that stress resistance—an organism’s ability to resist the effects of various types of stressors—peaks in early adulthood and declines with age. Interestingly, early adulthood also is the peak reproductive period for C. elegans. Coupled with evidence gathered from studying which molecular pathways are activated upon exposure to stress, these findings suggest the existence of genetic switches that shut down key stress resistance pathways following this important period in an organism’s life.
At the same time, additional results demonstrated that the accumulated damage that occurs during aging isn’t enough to activate stress response pathways. Rather, degradation of these vital response mechanisms may be due to hyperfunction, or the inability to shut down processes required for growth and other tasks.
What’s the takeaway?
Aging (and the breakdown of normal processes that comes with it) is a major component in many diseases, including Parkinson’s and Alzheimer’s diseases. Likewise, stress plays a key role in the onset of many other health issues. A better understanding of how the aging process occurs and how this genetic switch leads to decreased stress resistance may one day allow scientists to develop ways to keep the switch on, giving people more healthy years.
Why C. elegans?
C. elegans provides an excellent model for studying aging and neurodegenerative diseases thanks to it simple and well-mapped nervous system and ease of maintenance. Insights garnered from studying C. elegans often can be directly correlated in human disease.
